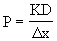
PERMEABILITY OF MOLECULES
Introduction: In the late 1800's, E. Overton discovered that substances that dissolve in lipids pass more easily into the cell than those that dissolve in water. This was some of the first evidence that cells were surrounded by a lipid membrane. The phospholipid membrane of cells can greatly modify the permeation of molecules into a cell. The membrane acts as a barrier to passive diffusion of water-soluble molecules. Howeve r, substances that dissolve in lipids pass more easily into the cell. The correlation between permeability and solubility in lipidly is appropriately named Overton's Rule.
Importance: The rate of diffusion into a cell (see DIFFUSION THROUGH THE CELL MEMBRANE) is related to the substrate's concentration, as well as properties of the substrate that determine its permeability. We can use an equation relating permeability to properties of the diffusing molecule to see how well permeability correlates with the dissolvability of molecules in nature.
Question: How is the permeability of a molecule across the lipoprotein membrane related to the molecule's solubility in lipids and size?
Variables:
|
P |
permeability coefficient for a particular substrate (cm/sec) |
|
K |
partition coefficient |
|
D |
diffusion coefficient (cm2/sec) |
|
D x |
width of the cell membrane (cm) |
Method: Permeability (P) of molecules across a membrane can be expressed as
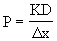
where K is the partition coefficient, D is the diffusion coefficient, and Dx is the thickness of the cell membrane. The diffusion coefficient (D) is a measure of the rate of entry into the cytoplasm depending on the molecular weight or size of a molecule. K is a measure of the solubility of the substance in lipids. A low value of K describes a molecule like water that is not soluble in lipid.
Graphically, we expect permeability (P) as a function of the partition coefficient (K) to increase linearly when D and Dx are constants. If we collect data for a variety of substances, we can plot permeability against partition coefficients to see how well the data fit our equation. Since the values are very small (ranging from 0.000005 to 4.0), we plot the x and y axes on log scales.
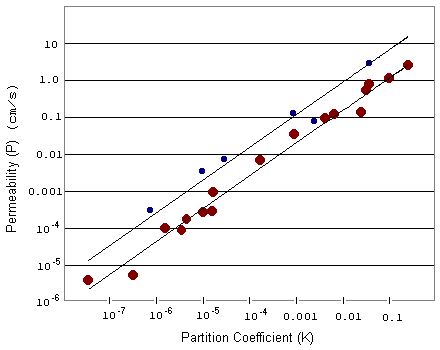
Interpretation: By plotting the data, we see that P does linearly increase with K, as described in our original equation. The small blue circles describe smaller molecules while the large red circles describe larger molecules. For a particular partition coefficient, the permeability of a smaller molecule is generally higher than that of a larger molecule.
Let's look again at the equation for permeability. When we plot P as a function of K, the slope of this line is given by D/Dx. Since smaller molecules generally penetrate more easily through the cell membrane, the diffusivity (D) is higher for smaller molecules. Thus when we plot P as a function of K for small and large molecules, we expect the line for the smaller molecules to have a larger, or steeper, slope. This is more easily seen when we plot the above data on a regular scale.
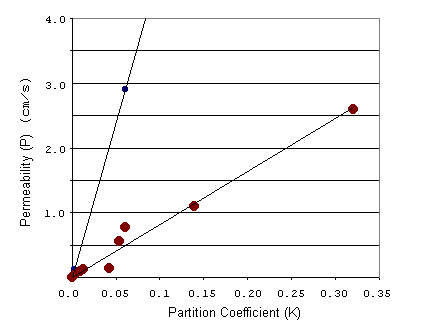
Indeed we find that the line for the smaller molecules has a much steeper slope attributed to the greater diffusion coefficient of smaller molecules.
Conclusion: The permeability of molecules through a cell membrane can be well described as a linear function of the partition coefficient with slope dependent on molecule size. For molecules of equal size, the one with greater solubility in lipids will pass more quickly into the cell. For molecules of equal solubility, smaller ones penetrate faster.
Additional Questions:
1. For a particular molecule with fixed diffusion and partition coefficients, how does permeability change as the width of the cell membrane decreases?
2. How might you expect the above graphs to change if we do the same experiment in cells with a much thicker membrane?
Sources: Walter, A. and J. Gutknecht. 1986. Permeability of small nonelectrolytes through lipid bilayer membranes. Journal of Membrane Biology 90:207-217.
Diamond, J. M. and Y. Katz. 1974. Interpretation of nonelectrolyte partition coefficients between dimyristoyl lecithin and water. Journal of Membrane Biology 17:121-154.
Copyright 1999 M. Beals, L. Gross, S. Harrell