COORDINATING PHOTOSYNTHETIC ACTIVITY: CIRCADIAN RHYTHMS
Introduction: The ability of organisms to measure time is evidence that they are well adapted to the 24-hour day/night cycle of our planet. Circadian rhythms are the daily repeating patterns of many organisms, such as the closing of flowers at night or opening of stomata during the day. An interesting property of circadian rhythms is that many are endogenous, arising from an internal biological clock. For example, in 1729, a French scientist discovered that circadian movements were not dependent on the daily cycling of light and dark, even though they were synchronized with it.
Photosynthesis is an important circadian rhythm in plants. Photosynthesis is the process by which plants convert carbon dioxide and water to sugar and oxygen:
6CO2 + 6H2O + light energy --> C6H12O6 + 6O2
Tiny openings in the leaves, called stomata, open and close to regulate the amount of CO2 taken in, and the amount of water and O2 exiting. The two pathways of photosynthesis, photophosphorylation and the Calvin-Benson Cycle, proceed within the chloroplast. The products of photophosphorylation, ATP and NADPH + H+, are converted to sugars in the Calvin-Benson Cycle. Because photophosphorylation requires light energy to proceed, both pathways of photosynthesis stop in the dark. It is therefore beneficial for plants to coordinate their photosynthetic activity and stomata activity with daily changes in light availability. The internal clock coordinating photosynthesis produces a circadian rhythm.
Importance: Photosynthesis dominates the metabolism of green plant cells. Therefore it is not surprising that many other plant activities, such as the opening of stomata or the activity of Photosystem II, are synchronized with the rhythm of photosynthesis. Because these rhythms are synchronized, however, it can be difficult to separate factors that are controlling the photosynthetic rhythm from those that are merely synchronized with it.
Scientists learn more about the circadian rhythms of photosynthesis by manipulating organisms and their environment, then measuring the corresponding changes in rhythm. Because circadian rhythms are repeating patterns, we can use principles of trigonometry to quantify them.
Question: How can we use trigonometry to quantify circadian rhythms of photosynthesis? What properties of the environment or plant are responsible for the photosynthetic rhythm? How do manipulations of light and carbon dioxide affect photosynthetic rhythms?
Variables:
|
y |
some property having a circadian rhythm such as carbon assimilation or percent of flowers opening |
|
t |
time (hours) |
|
A |
amplitude (same units as 'y') |
|
p |
period (hours) |
|
st |
phase shift in time (hours) |
|
sy |
phase shift in the value of 'y' (same units as 'y') |
Methods: Circadian rhythms produce repeating patterns over time. In general, we can describe any repeating rhythm by a sine wave:
y = sin t
where y is some property of the organism that exhibits a rhythm and t is time.

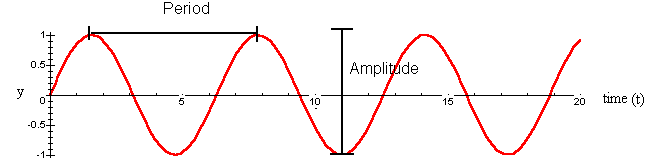
We can quantify properties of waves such as the amplitude and period. The amplitude of a wave is the height from peak to crest. For the simplest sine wave, y = sin t, the amplitude is 2. The period of a wave is the time for completion of one cycle. For y = sin t, the period is 2p.
Circadian rhythms are somewhat more complicated than the simplest sine wave. Instead of 2p, the period is 24 hours. The amplitude depends on what property of the plant we are considering. Additionally, environmental factors can affect the amplitude, period, and phase. Shifts in phase occur if the cycle begins sooner or later than normal, or if the peak and crests of the wave are higher or lower than normal. We can describe circadian rhythms by the following equation:
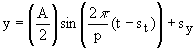
where A is the amplitude, p is the period, and sy is a phase shift in the height of y compared to the simplest sine wave (y = sin t). sy can be thought of as the value of y half way between the peak and crest of the wave; for the simplest sine wave sy = 0. st is a phase shift in time. For example, we could manipulate the circadian rhythm of flower opening by artificially creating a 24 hour day-night cycle 6 hours later than normal day and night. At time t = 0, the plant is moved to the artificial environment. Eventually the circadian rhythm of the plant's photosynthesis would shift to match the artificial day and night. The sine wave equation would be the same, except now st = 6 hours. The thin black line is a normal 24 hour day-night cycle, peaking at noon and cresting at midnight. The artificial cycle peaks and crests 6 hours later.
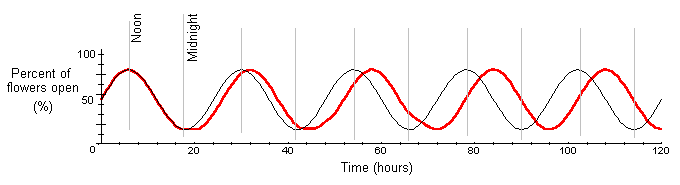
Circadian rhythms can be entrained through manipulative experiments to differ from a 24 hour cycle. For example, we could artificially create a day-night cycle of 22 hours. At time t = 0, the plant is placed in the artificial environment. Eventually, the rhythm of photosynthesis would change to a 22 hour period (p = 22). The graph shows a reduction in period from 24 to 22 hours when a plant is entrained to an artificial day-night cycle. The thin black line shows a normal 24 hour day-night cycle.

Under normal conditions, photosynthesis and stomatal opening have circadian rhythms. Here, photosynthesis is measured as carbon assimilation and stomatal opening is measured as conductance to water vapor. For the red kidney bean plant, data shows carbon assimilation varies between 5.8 and 8.2 mmol CO2 per m2 per second, with an amplitude is approximately 2.4 (A = 2.4). The period is approximately 24 hours (p = 24). We can approximate the normal circadian rhythm of photosynthesis as
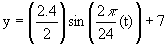
We can graph the circadian rhythm of photosynthesis over time. The graph of stomatal conductance, the lower curve, is also given, with coordinates on the second axis.
. 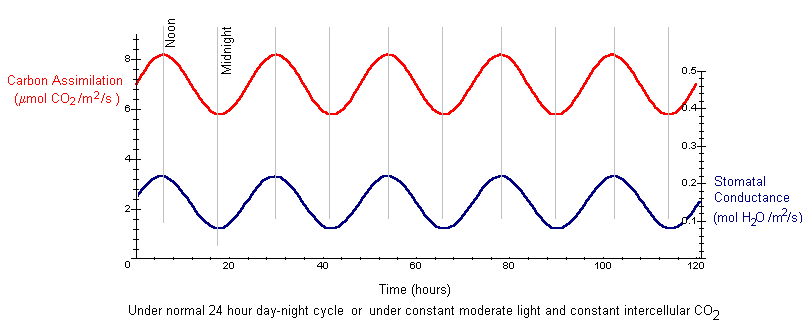
Interpretation: Under a normal 24 hour day-night cycle, both carbon assimilation and stomatal conductance have circadian rhythms. Both tend to be highest at noon, when light energy is highly available, and lowest at midnight.
If changes in light are driving the circadian rhythms, we might expect the sine waves to flatten when a plant is moved to constant light. However, when red kidney bean plants are moved from normal light (day and night every 24 hours) to constant moderate light, the circadian rhythm persists for several days with the same amplitude and period as the above curves. This indicates that changes in light and dark are not driving the circadian rhythm. Instead the rhythm is driven by an internal clock.
The opening and closing of stomata control the concentration of carbon dioxide inside the leaf. When stomatal conductance is high, a larger amount of CO2 can enter the leaf and be available for photosynthesis. Consequently, we might hypothesize that the circadian rhythm in stomatal conductance is causing the rhythm in carbon assimilation. If we manipulate a leaf to have constant levels of CO2, we might expect the rhythms of stomatal conductance and carbon assimilation to disappear. However, under constant moderate light (200 mmol per m2 per second photon flux density) and constant intercellular CO2 (28 Pa), the circadian rhythms still persist for several days with the same amplitude and period as under normal conditions (the curves in the previous graph).
Since photosynthesis is dependent on light energy, we might expect to see changes in circadian rhythm depending on the intensity of light. Under conditions of high light and high CO2, photosynthetic activity is very high. We can determine how such environmental conditions affect the circadian rhythm.
The graph below shows data from a single kidney bean leaflet for the circadian rhythms in carbon assimilation and stomatal conductance under constant high light (500 mmol per m2 per second photon flux density) and constant ambient CO2 (35 Pa).
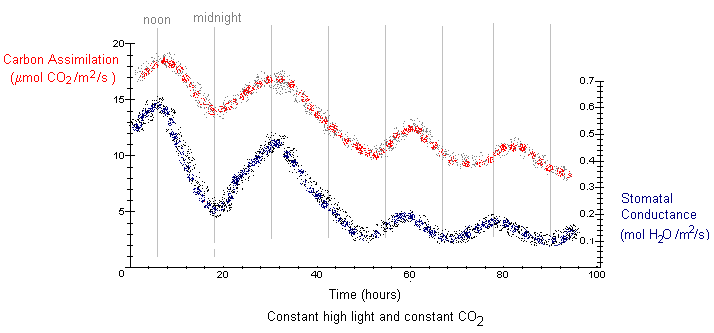
Under conditions of constant high light, the circadian rhythms in carbon assimilation and stomatal conductance quickly flatten out after a couple of days. The period is slightly longer than 24 hours. Notice that on the first day, the values of carbon assimilation (sy = 17 mmol CO2 per m2 per second) and stomatal conductance (sy = 0.52 mol H2O per m2 per second) were much higher than the values under moderate light conditions (sy = 7 and sy = 0.15, respectively). Under high light conditions, these values decrease over time, along with a decreasing amplitude. This is quite different from constant levels of moderate light, where the circadian rhythms persist for several days with the same amplitude and period as a 24 hour night-day cycle.
Under high light conditions, photosynthetic activity is very high. Therefore it is not suprising the initial values of carbon assimilation and stomatal conductance were much higher than leaflets in moderate light. Under conditions favorable to high photosynthetic activity, carbohydrate quickly accumulates in the leaves. The resulting decline in carbon assimilation and stomatal conductance over time may have occurred as starch accumulated in the leaves, disrupting chloroplast structure.
Conclusions: The cellular clock of plant cells is well adapted to the 24 hour day-night cycle of the planet. Circadian rhythms arise in various aspects of photosynthesis, including carbon assimilation, stomatal conductance, and in levels of essential elements of photosynthesis, such as Photosystem II and RuBP. Changes in light intensity and carbon dioxide concentration can affect circadian rhythms of photosynthesis.
Circadian rhythms are essentially sine waves with periods of 24 hours and variable amplitudes. Quantifying properties of circadian rhythms, such as amplitude and period, can help us understand the mechanisms controlling rhythms.
Additional Questions:
1. In the general sine equation for a circadian rhythm, what is the y-intercept if st = 0? What is the biological interpretation of the y-intercept?
2. Approximate the equation for stomatal conductance under normal conditions by estimating A, p, and sy. One cycle is completed every 24 hours. The highest conductance is 0.22 mol H2O per m2 per second and the lowest is 0.08. There is no phase shift in time, and the value of stomatal conductance is 0.15 at time t = 0.
Sources:
Hennessey, T. L. and C. B. Field. 1991. Circadian rhythms in photosynthesis: Oscillations in carbon assimilation and stomatal conductance under constant conditions. Plant Physiology 96:831-836.
Hennessey, T. L., A. L. Freeden, and C. B. Field. 1993. Environmental effects on circadian rhythms in photosynthesis and stomatal opening. Planta 189:369-376.
Mansfield, T. A. and P. J. Snaith. 1984. Circadian rhythms, p. 201-218 in Advanced Plant Physiology. M. B. Wilkins, ed. Pitman Publishing Limited, Marshfield, MA
Copyright 1999 M. Beals, L. Gross, S. Harrell