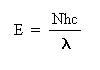
ENERGY ABSORBED FROM LIGHT
Introduction: Photosynthesis is the process by which light energy is converted to chemical energy. In plants, light is absorbed by pigments suchs as chlorophyll. Light hits these molecules and raises them to a higher energy. Light, however, occurs in a spectrum of wavelengths ranging from ultraviolet to visible light to infrared. The energy accompanying the absorption of light is dependent on the wavelength of the light.
Importance: The amount of energy absorbed during the processes of photosynthesis depends on the wavelength of light absorbed. For processes requiring more energy, pigment molecules must be able to absorb the necessary wavelengths.
Question: How is the amount of energy in a photon related to the wavelength of light?
Variables:
|
E |
energy in a mole of photons (cal) |
|
N |
Avogadro’s number (photons per mole) |
|
h |
Planck’s constant (cal/sec) |
|
c |
velocity of light (nm/sec) |
|
l |
wavelength of light (nm) |
Methods: The energy in a mole of photons can be related to the wavelength of light by the following equation:
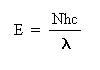
where E is the energy in a mole of photons, N is Avogadro's number (6.02 x 1023 photons per mole), h is Planck's constant (1.58 x 10-34 cal/s), c is the velocity of light (3 x 1017 nm/s), and l is the wavelength of light (nm).
This equation can be used to determine the amount of energy absorbed when photosynthesis occurs with lights of different wavelength. We will plot energy (E) as a function of wavelength (l).
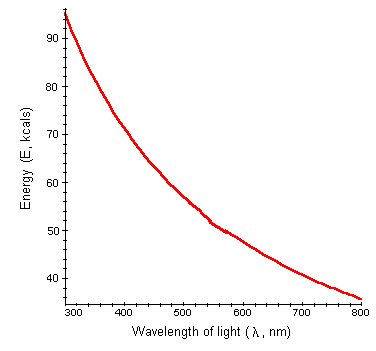
Interpretation: Energy as a function of wavelength is a hyperbolic function. Notice that as wavelength decreases, the energy absorbed increases. For example, red light has wavelength of 680 nm and contains a considerable amount of energy, 42 kcal. However, a pigment molecule absorbing blue light, with a wavelength 400 nm, absorbs about 71 kcal of energy.
Conclusions: The shorter the wavelength, the greater the energy absorbed. The different pigment molecules of plants absorb different wavelengths of light and consequently absorb different energies. Additionally, during photosynthesis, certain processes require higher energy than others. For example, photosystem II requires photons with slightly higher energies than photosystem I. As a result, chlorophyll molecules in photosystem II absorb light maximally at 680 nm while molecules in photosystem I can absorb light up to 700 nm.
Additional Questions:
1. Ultraviolet light has a wavelength of 284 nm. Would you expect it to be more or less energetic than visible light (400-700 nm)?
2. The frequency of light (n) is related to wavelength (l) by the following equation:
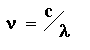
where c is the speed of light (cm/sec). Substitute this into the equation for energy. How is energy related to frequency of light? In other words, what kind of function is energy (E) as a function of light frequency (n)? Graph this function.
Source: Darnell, J., H. Lodish, and D. Baltimore. 1986. Molecular Cell Biology. Scientific American Books, Inc., New York
Purves, W. K., G. H. Orians, H. C. Heller, and D. Sadava. 1998. Life: The Science of Biology (Fifth Edition). Sinauer Associates, Sunderland, MA.
Copyright 1999 M. Beals, L. Gross, S. Harrell