NASTIC MOVEMENTS IN PLANTS
Introduction: Plants are capable of rapidly adapting to changes in their environment. This can be in the form of irreversible changes in growth (See Cell Enlargement) or reversible movements in response to a stimulus. Nastic movements in plants are reversible and repeatable movements in response to a stimulus whose direction is determined by the anatomy of the plant. Examples include the diurnal movement of leaves and the response of insectivorous plants, such as the Venus fly trap, to prey. Nastic movement is generally caused by elastic changes in the size of special motor cells within the plant tissue. These changes are generally produced by changes in osmotic pressure due to an influx or efflux of ions that cause water to move in or out of the cells. In many plants, shrinkage of the motor cells causes the overall movement of the plant.
Importance: We can use a model to determine if nastic movements are generally driven by osmosis. If osmotic pressure is the main factor driving nastic movements, then rates of movement in plants should match those predicted by an osmotic model. This is particularly important for cases such as insectivorous plants where movement is very rapid and seems unlikely to be driven solely by osmosis.
Question: How are nastic movements in plants affected by changes in osmotic pressure? Are rapid nastic movements consistent with an osmotic driven model?
Variables:
|
Jv |
change in cell volume (mm3/sec) |
|
Lp |
hydraulic conductivity of cell membrane (m sec-1 Pa-1) |
|
D P |
difference in internal and external hydrostatic pressure (Pa) |
|
Dp |
difference in internal and external osmotic pressure (Pa) |
|
T1/2 |
half time for volume change (sec) |
|
V |
cell volume (mm3) |
|
A |
cell surface area (mm3) |
|
e |
cell volume elastic modulus (Pa) |
|
p I |
internal osmotic pressure (Pa) |
Methods: We can derive a model for the rate of nastic movement based on the osmotic pressure inside a cell. For a single plant cell with a semi-permeable membrane, the change in volume depends on the hydraulic conductivity of the membrane (Lp) and the hydrostatic (DP) and osmotic pressure (Dp) differences across the cell membrane. We can write this as
Jv = Lp (DP-Dp)
where Jv is the volume flux. For small changes in Dp, Jv is an exponential function.
A convenient way to look at rates of nastic movement is to examine the time it takes for a cell to shrink to half its original volume. We can derive the following equation
![]()
where T1/2 is the half time for volume change, A and V are the surface area and volume of the cell, Lp is the hydraulic conductivity of the membrane, e is the volume elastic modulus of the cell, and pi is the internal osmotic pressure.
Osmotic pressure (pi) reflects the the pressure required to stop the net flow of water across the plant cell membrane. Osmotic pressure is high when solute concentration is high; water flows in the direction of high solute concentration in order to maintain equal osmotic pressure on both sides of a membrane. When the osmotic pressure inside of the cell (pi) is larger than that outside of the cell (po), water flows into the cell.
We can use the osmotic model for T1/2 to determine whether experimental rates of nastic movement, particularly fast movement, are consistent with being driven by osmotic pressure. According to our model, a cell must experience some change in size, osmotic pressure, or membrane conductivity in order to achieve a fast rate of movement. For normal cells, we expect V and A and to be fairly consistent. Therefore, we will plot T1/2 as a function of Lp for two different values of e+pi, 4x106 Pa and 6x106 Pa. We take V = 20000 mm3, A = 5000 mm2 for a cell of size 50mm x 20mm x 20mm .
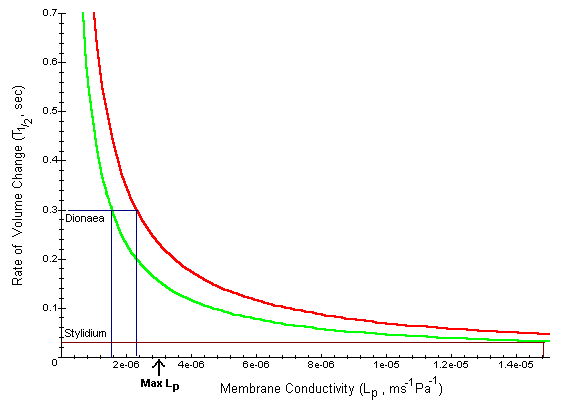
Notice that T1/2 as a function of Lp is a hyperbolic function. As the membrane conductivity increases, the amount of time for the cell to shrink in size is greatly reduced. The top line is e+pi = 4x106 Pa. As the volume elastic modulus increases (e+pi= 6x106 Pa), this curve shifts to the left. This implies that the cell shrinks much faster as the elasticity of their cell walls increases.
Interpretation: Drosera is an insectivorous plant whose modified leaves are covered with sticky, hair-like structures. When an insect becomes stuck to one of the hairs, the leaves slowly close to entrap it. The movement takes up to 3 minutes, and T1/2 is a few seconds. By looking at the graph, we see a membrane conductivity (Lp) of 2.5 x 10-7 to 3.5 x 10-7 is required to accommodate this rate of movement. For Drosera, nastic movements may certainly be driven by osmotic pressure.
The Venus fly trap, Dionaea, closes on its prey much more rapidly, approximately 300 milliseconds. In order for this rate of movement to be explained by the osmotic model, we must have an increase in Lp(e+pi) of 10 to 20 times. Although values of Lp are unlikely to be much greater than 3x10-12 m/(s Pa), it is possible for e to increase as cells surrounding the motor cell serve to stiffen up the tissue. This is similar to our graph where higher values of e+pi shift the curve to the left, so lower values of Lp are required.
The plant Stylidium has a pollination mechanism by which it flips its reproductive structures when stimulated by nectar gathering insects. The structure flips through an angle of up to 4 radians in 10 to 20 ms. In order for this rate of movement to be explained by the osmotic model, we must have an increase in Lp(e+pi) of 100 to 200 times. It is highly unlikely that Lp or e could be high enough to support such a high rate of movement. Therefore, the fast movement of Stylidium appears to be at too high a rate to be driven by osmosis. Another mechanism, such as the collapse of specialized cells, may be responsible for the fast movement.
Conclusions: Nastic movements are generally caused when a stimulus causes electrical properties of the plant cell to change rapidly, causing a change in osmotic pressure. By using a simple model of osmotic driven movement, we can distinguish cases where osmosis is insufficient to drive movement.
Additional Questions:
1. For internodal cells of Nitella flexilis, the cell dimensions are 50mm x 20mm x 20mm, Lp=1x10-12 m/(s Pa), and e+pi=5x106 Pa. Calculate the half time for volume change (T1/2). (You will need to know how to calculate the volume and surface area of a cube).
2. According to the osmotic model, how is the rate of nastic movement affected by changes in surface area (A)? Biologically, why might this be? (Compare cells of similar volume, but one is shaped like a sphere and the other like a thin cyllinder.)
Sources: Findlay, G. P. 1984. Nastic Movements. Pages 186-200 in Advanced Plant Physiology, Edited by M. B. Wilkins. Pitman Publishing, Marshfield, MA
Dainty, J. 1976. Water relations of plant cells. Pages 12-35 in Encyclopedia of Plant Physiology, New Series, vol. 2. Part A. Edited by U. Luttge and M. G. Pitman. Springer-Verlag, NY, NY.
Hill, B. S. and G. P. Findlay. 1981. The power of movement in plants: The role of osmotic machines. Quarterly Review of Biophysics 14:173-222.
Copyright 1999 M. Beals, L. Gross, S. Harrell