NUCLEAR AND CYTOPLASMIC VISCOSITY
Introduction: The membrane of the nucleus is perforated by nuclear pores that permit the entry of water soluble molecules from the cytoplasm into the nucleoplasm. The viscosity of the nucleus and the cytoplasm can affect the rate at which molecules flow through cellular fluid.
Importance: We certainly expect liquid inside a cell to be somewhat viscous in order to support the organelles. But how does the viscosity of the nucleus compare to the cytoplasm? We can experimentally determine the viscosity of the cytoplasm and nucleoplasm by looking at the diffusion of molecules in the cytoplasm and nucleoplasm and relating this to a simple equation describing diffusion.
Question: How does the viscosity of the nucleoplasm compare to the viscosity of the cytoplasm?
Variables:
|
D |
rate of diffusion of molecules (micrometer2/sec) |
|
b |
a constant (micrometer2/sec) |
|
k |
Boltzmann's constant (1.3807 x 10-18(g micrometers2)/(s2 K)) |
|
T |
the absolute temperature (K) |
|
v |
viscosity (g/(cm s)) |
|
r |
radius of diffusing molecule (cm) |
Method: The viscosity of the cytoplasm and the aqueous phase of the nucleus can be deduced using a modified Stokes-Einstein equation. Diffusion of molecules through cellular fluid can be estimated as
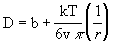
where k is Boltzmann's constant, T is the absolute temperature, v is the viscosity, r is the radius of the diffusing molecule, and b is a constant that gives the y-intercept when D is graphed as function of r. Diffusivity of different sized molecules in the cytoplasm and the nucleus can be measured experimentally when temperature is held constant. We can experimentally measure the diffusivity of different sized molecules in a dilute solution, cytoplasm, and nucleoplasm. As the molecule radius decreases, we expect diffusivity to increase. The experimental data can be plotted as increasing functions of 1/r. Viscosity is then reflected in the slope of the plotted data and can be calculated from the above equation (slope = kT/6vp) as the value that best fits the data.
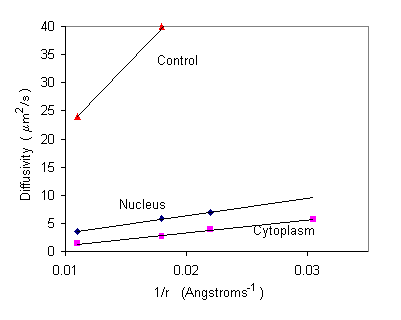
Interpretation: From the graph, we can see that the slope of the control (a dilute solution) is much steeper than that of the cytoplasm or nucleoplasm. From the modified Stokes-Einstein equation, we know as viscosity increases, slope decreases (slope = kT/6vp). Additionally, the slope of the nucleoplasm slightly steeper than that of the cytoplasm. We can interpret this as higher viscosity in the cytoplasm. In the cells graphed above, the cytoplasm was about 1.2 times more viscous than the nucleoplasm and 8.1 times more viscous than the control.
Conclusion: The Stokes-Einstein equation is not only a good model of how diffusivity is related to properties of the molecule and solution. This equation can be used to experimentally assess properties of the cell cytoplasm and nucleus.
Additional Questions:
1. Look at the equation for diffusivity. Assume the molecule radius and viscosity are held constant. How does diffusivity change with increasing temperature?
Source: I. Lang, M. Scholz, and R. Peters. 1986. Molecular mobility and nucleocytoplasmic flux in hepatoma cells. Journal of Cell Biology 102:1183-1190
Copyright 1999 M. Beals, L. Gross, S. Harrell