
OSMOREGULATION
Introduction: Molecules passively diffuse from regions of high to low concentration. Aquatic animals are generally hyperosmotic to their surroundings: their internal solute concentration is much higher than their surroundings. Because of this, aquatic animals must develop physiological mechanisms to prevent excess flow of water into their bodies. They must also develop mechanisms to prevent the loss of solutes as excess water is excreted. The process by which organisms actively maintain their internal solute concentration is called osmoregulation. Animals must actively transport solutes from their surroundings into their blood against the concentration gradient.
Importance: The work required for osmoregulation depends on properties of the organism and its environment. Some animals seem to spend more energy than others to maintain an internal balance. We can quantify osmotic work in a simple equation in order to understand tradeoffs organisms must make in order to maintain their internal solute concentration.
Question: How is osmotic work related to properties of the organism?
Variables:
|
W |
osmotic work (cal/hr) |
|
R |
universal gas constant (0.00199 cal/(mmol K)) |
|
T |
external temperature (K) |
|
V |
volume of urine (mL/h) |
|
Cu |
solute concentration in the urine (mmol/mL) |
|
Cb |
solute concentration in the blood (mmol/mL) |
|
Cm |
solute concentration in surrounding medium (mmol/mL) |
Methods: Assuming the animal is only permeable to water, the energy required for osmoregulation can be described by an equation. We assume the work required to maintain the internal solute concentration is related to the solute concentration in the blood (Cb) and environment (Cm), temperature (T), and the amount of solutes lost in urine (V´ Cu):

We can plot W as a function of Cb/Cm to understand how the concentration of solutes in the blood and surroundings affects the osmoregulation of an organism.
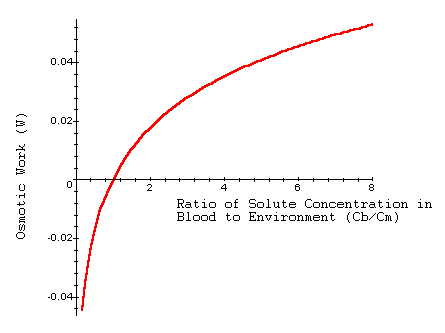
Interpretation: Here, osmotic work (W) involves active transport of ions into the organism to prevent the internal concentration from becoming too diluted when Cb > Cm. Notice that when Cb < Cm, water moves out of the organism. In this case, we are taking the natural log of a number less than 1, so the osmotic work is negative. This implies an organism must actively work to prevent the internal solute concentration from becoming too high as it loses water (opposite of the hyperosmostic situation).
By looking at the equation for osmotic work, we can determine what properties of an organism could reduce the amount of work required for osmoregulation. Potts (1954) collected the following data for three similar sized aquatic organisms.
|
Animal |
Blood Concentration (mmol/mL) |
Urine Concentration (mmol/mL) |
Urine Volume (mL/hr) |
Metabolic rate (cal/hr) |
|
Mitten crab |
.320 |
.320 |
.1 |
14 |
|
Crayfish |
.420 |
.124 |
.1 |
10 |
|
Clam |
.042 |
.024 |
.5 |
1.2 |
One way to reduce work is to reduce the concentration of solutes in the urine (Cu). This means an animal like the crayfish, that reabsorbs solutes from urine, has a considerable energy savings. For animals like the mitten crab with high solute concentrations in the blood or who lose a large amount of solutes through urine, osmotic work will be quite high. In other words, a high metabolic rate is necessary to maintain a stable internal state. Similarly, animals with low metabolic rates must maintain low osmotic work. An animal like the clam, who loses a high volume of urine (V), must maintain dilute blood (Cb) and urine (Cu) in order to survive.
Conclusions: Many aquatic animals must expend energy in order to maintain an internal ionic balance. Animals, however, differ in mechanisms they utilize to minimize osmotic work. Quantifying osmotic work can help us understand these properties.
Additional Questions:
1. Calculate osmotic work for the data from Potts using the equation for osmotic work. (R = 0.00199 cal/(mmol K), T = 300 K, Cm = .006mmol/mL).
2. What happens to osmotic work (W) as temperature (T) increases? What can an organism do to meet the new energy need?
Sources: Potts, W. T. W. 1954. The energetics of osmostic regulation in brackish and freshwater animals. Journal of Experimental Biology 31:618-630.
Schmidt-Nielsen, K. 1990. Animal Physiology: Adaptation and environment. Cambridge University Press, Cambridge
Copyright 1999 M. Beals, L. Gross, S. Harrell