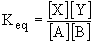
Introduction: Water is the universal solvent inside all cells and extracellular fluids. Water molecules (H2O) can dissociate into hydroxide ions (OH-) and hydrogen ions (H+). Other molecules or parts of molecules have the ability to either give up hydrogen ions, acids, or accept hydrogen ions, bases. Consequently, we can characterize any aqueous solution by the concentration of positively charged hydrogen ions and negatively charged hydroxide ions.
Importance: Many chemical reactions in living cells involve exchanges of hydrogen ions. Because changes in acidity can affect both the structure and chemical reactivity of cellular molecules, cells must constantly maintain an acid-base balance.
Questions: How do we quantify acidity? What affects the buffering capacity of acids and bases?
Variables:
| Keq | equilibrium constant |
| [X], [Y] | concentrations of reactants |
| [A], [B] | concentrations of products |
| d | fraction of a weak acid that is undissociated |
Hydrogen ion concentration and pH
Methods:
The dissociation of water into hydroxide and hydrogen ions can be represented by the following reversible chemical equation:
H2O ---> H+ + OH-
The hydrogen ion is short-lived and combines with an H2O molecule to form a hydronium ion (H3O+).
The equilibrium constant of a chemical reaction is given by the ratio of products to reactants at equilibrium. For the general chemical equation X + Y ---> A + B, we can write the equilibrium constant as
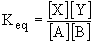
For the dissociation of water we get
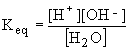
The equilibrium constant for the dissociation of water at 25° C has been measured as Keq=1.8x10-16. This number is small because only a small fraction of water molecules dissociate. The concentration of water can be determined from the fact that 1 mole of water weighs 18g and 1 liter of water weighs 1000g. Hence, the concentration of pure water [H2O] is 1000g/L ¸ 18 g/mol = 55.5 mol/L. By substituting these into the above equation, we find that
[H+][OH-]=1x10-14
In pure water, the concentrations of hydrogen and hydroxide ions are about the same. Hence by taking the square root of 1x10-14 we find that [H+] and [OH-] are each about 10-7 M. This means that 1 liter of pure water contains about one ten-millionth of a mole of hydrogen or hydroxide ions.
Other molecules, however, have the ability to donate or accept hydrogen ions. Consequently, when other substances are dissolved in water, the concentrations of H+ and OH- can change. As the concentration of hydrogen ions increases, the concentration of free hydroxide ions decreases in order to maintain an equilibrium.
A convenient way to characterize aqueous solutions is to look at the hydrogen ion concentration. The pH scale allows biologists to define chemical solutions more conveniently:
pH = -log[H+]
This method is convenient because it eliminates the need for exponential notation.
We can see how pH changes with hydrogen ion concentration by plotting the equation for pH as a logarithmic function of [H+].
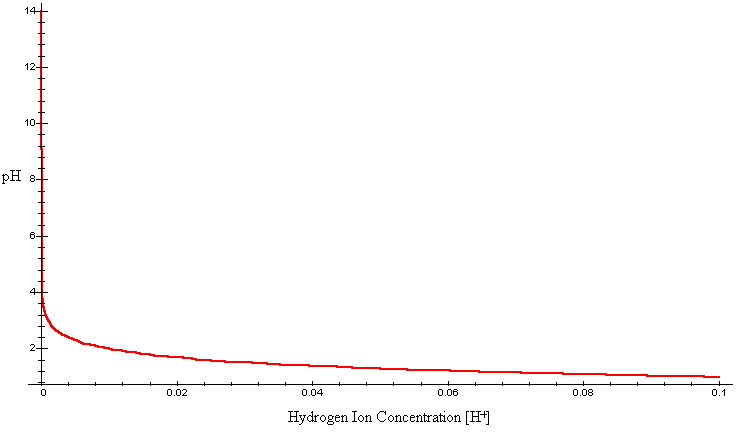
We can more clearly see how [H+] affects pH by plotting this graph on a semi-log scale. This will make it easier for us to see values of pH at very low values of [H+].
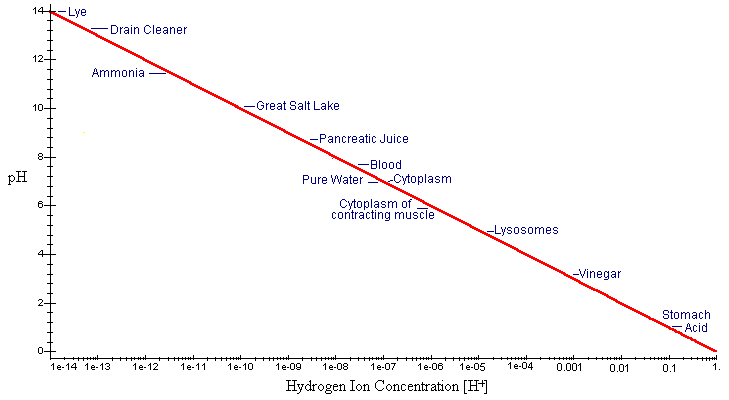
In the simplest terms, the value of pH simply gives us the absolute value of the exponent of the hydrogen ion concentration.
On the pH scale, a 7 is considered to be neutral. Substances that can donate hydrogen ions, thus increasing [H+], are acids. Strong acids have pH much lower than 7. Molecules that accept hydrogen ions, thus decreasing [H+], are bases and have pH higher than 7. Some examples are given in the graph.
Buffers
Introduction: Cells must constantly maintain their pH in order to function properly. In animals, for example, the maintainence of blood pH is crucial for life. A slightly acidic pH (6.95) would result in coma and death. A slightly more basic pH (7.7) would result in convulsions and muscle spasms. As another example, the pH of cellular organelles such as lysosomes (around 5) is lower than the pH of the cytoplasm (around 7.2). Lysosomes contain enzymes that function optimally in an acidic environment. Such an acidic environment, however, would be detrimental to biological processes in the cytoplasm. Each must maintain the appropriate pH.
Methods: Cells can maintain pH chemically by using buffers. Buffers are molecules that easily interconvert between acidic and basic forms, donating or accepting protons as conditions change. The dissociation of a simple acid HA can be described by the following chemical reaction:
HA ---> H+ + A-
We can interpret this as a weak acid (HA) dissociating to form a hydrogen ion (H+) and a conjugate base (A-). Notice the reverse happens as well. Buffer solutions are typically mixtures of a weak acid and its conjugate base. Suppose we were to add a strong base, NaOH, to the buffer solution. NaOH would raply dissociate into Na+ and OH- ions. The hydroxide ions would rapidly accept the H+ ions formed by the buffer. Similarly, if we added a strong acid, the conjugate base, A-, would take up the additional hydrogen ions. In either case, the buffer (HA) would then continue to dissociate in order to maintain its original equilibrium.
Buffering ability depends on the ratio of conjugate base concentration to weak acid concentration. Additionally, the degree of dissociation depends on the pH of the solution. We can understand this better by deriving the Henderson-Hasselbalch equation.
The equilibrium constant for the dissociation of a weak acid is given by
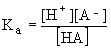
The Henderson-Hasselbalch equation is derived from this by taking the logarithm of both sides and rearranging to get
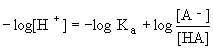
We can rewrite -log[H+] as pH and -logKa as pKa to get the following equation:
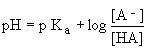
We can think of the pKa as the pH at which the number of molecules of conjugate base [A-] and weak acid [HA] are equal. Thus we get log[1] = 0 and pKa = pH. We can interpret the above equation in the following way: pH is a function depending on the constant, pKa, as well as the ratio of conjugate base to weak acid, [A-]/[HA].
By rearranging the Henderson-Hasselbalch equation slightly, we can calculate the degree of dissociation of an acid (d) if both the pH of the solution and the pKa of the acid are known:
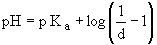
where d = [HA] / ([A-]+[HA]). In other words, d is the fraction of weak acid-conjugate base mixture that is undissociated weak acid. We will plot pH as a function of d for a pKa = 7.2.
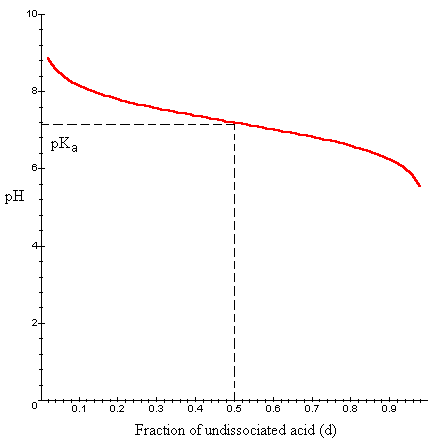
Interpretation: Suppose we have a solution of acid or base at its pKa value. This means the concentrations of weak acid and conjugate base are equal, or d = 0.5. Notice the curve around this value is relatively flat. If we add additional acid or base, the fraction of undissociated buffer would change in order to maintain an equillibrium. However, because the curve is relatively flat, the pH of the solution would increase or decrease only slightly. This ability of an acid or base to prevent the pH of a solution from changing drastically is called buffering.
We can see from the graph that the buffering ability of a weak acid or base depends on the fraction dissociated. The buffering ability is highest when the conjugate base [A-] and weak acid [HA] are present in equal concentrations (d = 0.5). If d is much higher or lower than 0.5, then additional acid or base will result in a more dramatic change in pH.
For example, phosphoric acid is physiologically important in cells. Part of the dissociation or phosphoric acid can be described by the following equation:
H2PO4- ---> HPO42- + H+
This dissociation has a pKa of 7.2. In an acidic solution, a high fraction of this acid would be undissociated (low concentration of conjugate base). We can see from the above graph, that if d = 0.9, the addition of acid or base would lead to a dramatic change in pH. In cells, however, phosphate ions are present in considerable quantities. Additionally, the pH of cellular cytoplasm is approximately 7.2. At this pH, H2PO4- has a very high buffering capacity. In fact, phosphate ions play an important role in maintaining, through their buffering ability, the pH of the cytoplasm.
Conclusions: Because water is the universal solvent for all life, it is important to know how its chemical properties can affect cells. One of the most important characterizations of a biological fluid is its pH. The concentration of hydrogen and hydroxide ions in an aqueous solution can greatly affect both the structure and chemical reactivity of cellular molecules. Cells are cell organelles must maintain an appropriate pH in order to function optimally. Additionally, dramatic shifts in pH can play a role in controlling cellular activities such as egg division after fertilization. Consequently, cells must work constantly to maintain an acid-base balance. At the appropriate pH and concentration, buffers can be highly important in maintaining pH by preventing drastic changes.
Additional Questions:
1. The pH of cellular cytoplasm is normally about 7.2. Cell organelles, such as lysosomes, have a much lower pH of around 5. What is the hydrogen ion concentration for each of these? How many times higher is the [H+] of lysosomes than of cytoplasm?
2. Use rules of logarithms to derive the Henderson-Hasselbalch equation.
Sources:
Darnell, J., H. Lodish, and D. Baltimore. 1986. Molecular Cell Biology. Scientific American Books, Inc., New York
Schwarz, O. J. 2000. Introduction to Basic Laboratory Skills for the Biological Sciences - An Experimental Approach. Performance Press, Oak Ridge, TN.
Tobin, A. J. and R. E. Morel. 1997. Asking About Cells. Harcourt and
Brace, Orlando, FL.
Copyright 1999 M. Beals, L. Gross, S. Harrell