Introduction: Many biological processes involve the aggregation of cells into a cluster. For example, in animals, small cells called platelets cluster at the site of an injury to the skin or blood vessels. Also, during the development of an embryo, space between aggregated cells decreases and cell-to-cell contact increases. The amount of space between aggregated cells can affect the permeability of solutes into the cells from the surrounding solution. The number of cell-to-cell contacts, places where the cell membranes of different cells touch, can affect flow of solutes and communication between cells.
Importance: The ability to objectively examine aggregations of cells requires a quantitative measure of the closeness of cells. We can use the geometrical principles of sphere packing to quantify the density of aggregated cells.
Questions: How does the arrangement of cells within a cluster affect the space between cells and the number of cell-to-cell contacts? How do biological examples of sphere packing compare to mathematical theory?
Variables:
| P | packing density |
| Vsphere | volume of spheres within a cube |
| Vcube | volume of a cube |
| r | radius of a sphere |
| l, w, h | length, width, height of a cube |
| R | ratio of aggregate volume to individual cell volume |
| Vagg | volume of cell aggregate |
| Vcell | volume of individual cell |
Methods: Sphere packing is a problem by which mathematicians try to determine how tightly spheres can be packed in a box. Imagine a grocer trying to pack oranges into a box or stack oranges in his store: his goal is to pack the oranges as tightly as possible. Randomly arranging the oranges may result in an inefficient use of the space.
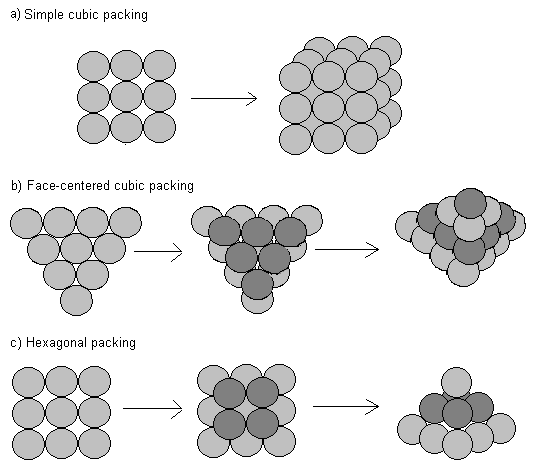
We will examine three different models from sphere packing theory: simple cubic packing, face-centered cubic packing, and hexagonal close packing. In simple cubic packing, spheres are stacked directly next to and on top of each other. In face-centered cubic packing, each layer has spheres placed diagonally next to each other. The next layer of spheres is placed in the crevices between spheres on the bottom layer. Every third layer directly overlies each other. In hexagonal packing, the bottom layer has spheres directly next to each other. Spheres in the next layer are placed in the crevices of the underlying layer. In hexagonal packing, every other layer directly overlies each other.

We can determine which of these formations is the most efficient use of space when packing spheres in a cube by calculating the packing density, or fraction of the cube's volume that is occupied by spheres. The packing density (P) is
P = Vsphere / Vcube
where Vsphere is the total volume of the spheres in a cube and Vcube is the total volume of the cube.
In simple cubic packing, each sphere is stacked directly on another sphere. We can visualize each sphere being contained within an individual cube.
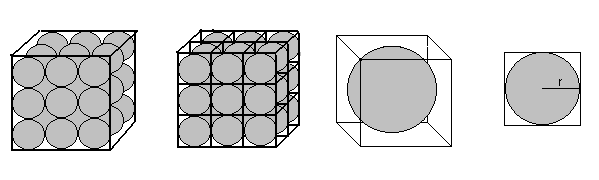
We can then calculate the packing density as the volume of a single sphere divided by the volume of a single cube. The volume of a sphere is given as
Vsphere = 4/3 p r3,
where r is the radius of the sphere. The volume of the cube is given as
Vcube = l w h,
or length times width times height. The width, height, and length of the cube are exactly equal to the diameter, or twice the radius of the sphere. So Vcube=(2r)(2r)(2r). We can therefore calculate the packing density as
P = Vsphere / Vcube = (4/3 p r3) / (2r)3
which is approximately P=0.524. In other words, 52.4% of the space in the cube is occupied by the sphere and 47.6% is empty space. For any number of spheres we stack in this formation, the density remains the same.
We can use a similar approach to determine the packing density for face-centered cubic packing. First, following the above procedure, we must determine how many spheres fill an individual cube. Visualize a group of 6 spheres placed in an equilateral triangle, with an additional sphere placed on top. Take another group of 7 spheres in the same formation, but oriented in the opposite direction. Now place each group of 7 back to back. A symmetrical cube emerges containing eight 1/8 spheres in each corner, and six 1/2 spheres at each face. Stacking additional cubes next to each other gives us the same packing arrangement of spheres. It is easiest, however, to calculate the packing density for a single cube.
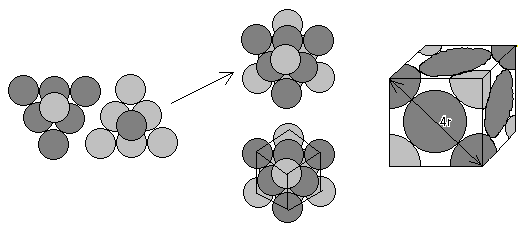
With face-centered cubic packing, the cube contains eight 1/8 spheres and six 1/2 spheres. Therefore, knowing the volume of a whole sphere is 4/3 p r3, we can calculate the volume of spheres within the cube as the volume of 1/8 of a sphere, times 8, plus the volume of 1/2 sphere, times 6:
Vsphere = 8 (1/8)(4/3 p r3 ) + 6 (1/2)(4/3 p r3 ) = (1+3)(4/3 p r3 ) = 16/3 p r3
where r is the radius of a single sphere.
We can also calculate the volume of the cube since we know the diagonal distance along the face of the cube is 4r, where r is the radius of a single sphere. In order to calculate the volume of a cube, however, we need to know the length of one side of the square (length = width = height). Since the diagonal and two sides of a square form a right triangle, we can use the Pythagorean Theorem to find the length of one side. In this case, l=w=h=2Ö 2r , and the volume of the cube is
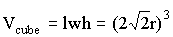
We can therefore calculate the packing density as
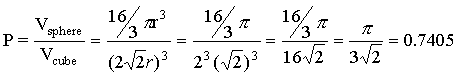
In other words, with face-centered cubic packing, 74.05% of the space is occupied by spheres and 25.95% is unoccupied space.
Hexagonal close packing is very similar to face-centered cubic packing, except spheres are shifted to a slightly different angle. Consequently, the packing density for hexagonal packing is also P=0.7405, but is more difficult to verify using simple geometry. Demonstrations with ball bearings in a box and computer simulations have shown that when spheres are packed randomly, the packing density is around P=0.64.
Interpretation: By relating sphere packing to the aggregation of individual cells, scientists can gain a better understanding of the packing density of cells. Mathematicians have found that the most efficient packing of spheres is that of face-centered cubic packing or hexagonal close packing, P=0.7405. The outside surface of many viruses is formed of proteins that are packed in this way. Mature yeast cells, however, have a packing density of about 78%. Yeast cell shape is a slight departure from spherical, allowing a slightly tighter packing. Since randomly packed spheres have a packing density of approximately 64%, this suggests there is some order in yeast cell aggregation to reduce the amount of space between cells or increase cell-to-cell contact.
The volume of a single cell can affect the density of aggregated cells and the amount of intercellular space. For an aggregate of cells in an extra-cellular solution with a high osmality (see Water Movement and the Vant Hoff Equation), water tends to flow out of the cells. As the volume of individual cells decreases, the packing density decreases and the space between cells increases. For aggregates of yeast cells submerged in solution, Arnold and Lacy (1977) showed that the space between cells increases, or the packing density decreases, with increasing osmality.
In embryo development, cells are packed tightly together. As cells differentiate and the embryo develops, the amount of space between cells decreases and the number of cell-to-cell contacts increases. In order to test hypotheses about embryo development, scientists must be able to count the number of cells in each aggregate for different treatments. Martin et al. (1997) use sphere packing theory to estimate the number of cells in an aggregate.
If the volume of an aggregate is completely filled with cells and contains no intercellular space, then the number of cells in an aggregate would be given by the ratio (R) of the total volume of the aggregate of cells (Vagg) to the volume of a single cell (Vcell):
R = Vagg/Vcell
To account for intercellular space, however, we multiply this ratio (R) by the fraction of the total volume occupied by cells, or the packing density (P). Therefore the number of cells in an aggregate (N) is given by N=RP.
Mathematicians know that face-centered cubic packing or hexagonal packing obtains one of the greatests possible densities of spheres, P = 0.7405. One of the looser packings of spheres is simple cubic packing, P=0.5235. Consequently, Martin et al. (1997) estimate the number of spherical cells in an aggregate by assuming their packing density lies between 0.5235 and 0.7405. By simply calculating R, the ratio of the total aggregate volume to the volume of a single cell, they can estimate the number of cells in an aggregate as
0.5235R < cell number < 0.7405R
This is a much quicker, more efficient, and reliable way to estimate cell number than by counting.
Sphere packing theory is also useful in testing the effects of different medications on platelet aggregation. When animals suffer damage to their skin and blood vessels, small cells called platelets cluster at the site of injury. In order to test the effects of anti-aggregating drugs, scientist must be able quantify how densely packed platelets are into aggregates.
Conclusions: Biologists often need measurements of cell aggregation
in order to understand aspects of biology such as embryo development, platelet
aggregation, and permeability. Sphere packing density allows scientists
to take a quantitative approach to examine cell aggregation. Mathematicians
can calculate packing densities for different sphere packing formations.
By comparing their experiments to mathematical models, biologists can determine
characteristics of individual cells and cell aggregates, test hypotheses
about the effects of drugs or other external factors, and understand the
nature of cell aggregation.
Additional Questions:
1. For simple cubic packing, why is the packing density for an individual sphere within a cube the same as the packing number for any number of spheres packed within a larger cube? For example, calculate the packing density (P) for a simple cubic packing of 27 spheres, and verify it is P=0.524.
2. Use the Pythagorean Theorem (the sum of the squares of each side, a and b, is equal to the square of the hypotenuse, c; a2 + b2 = c2) to verify the length of one side of the cube in face-centered cubic packing. Remember in a cube, length = width = height.
3. Using Martin et al.'s method, estimate an upper and lower bound for
the number of spherical cells in an aggregate of cells with volume Vagg=50mm3.
The volume of an individual cell is approximately Vcell=0.05
mm3.
Sources:
Arnold, W. N. 2000. From cannon balls to yeast cells. Science 288:55
Arnold, W. N. and J. S. Lacy. 1977. Permeability of the cell envelope and osmotic behavior in Saccharomyces cerevisiae. Journal of Bacteriology 131:564-571.
Martin, I., B. Dozin, R. Quarto, R. Cancedda, and F. Beltrame. 1997. Computer-based technique for cell aggregation analysis and cell aggregation in in vitro chondrogenesis. Cytometry 28:141-146.
Weisstein, E. W. 2000. http://mathworld.wolfram.com/SpherePacking.html
and references therein
Copyright 2000 M. Beals, L. Gross, S. Harrell